NEC Lawsuit: A Comprehensive Overview of 2024 Developments
Date Modified:
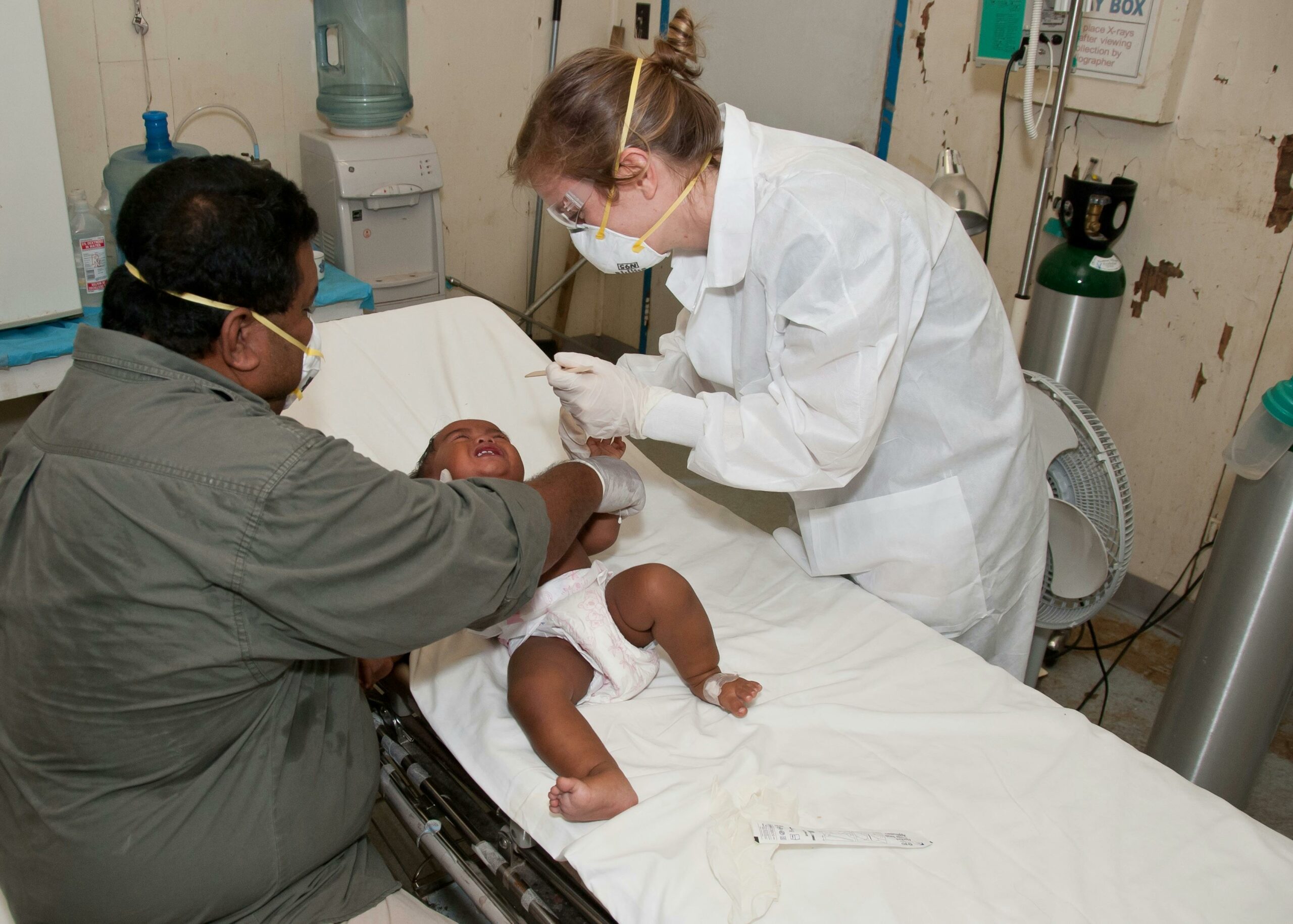
The NEC lawsuit involves allegations that certain baby formulas made from cow’s milk, specifically Similac and Enfamil, pose a risk of causing necrotizing enterocolitis (NEC) in premature babies. The litigation claims that the manufacturers, Abbott Laboratories and Mead Johnson, did not provide adequate warnings about this severe health risk.
Consequently, numerous families whose infants developed NEC after consuming these formulas have initiated legal actions against the manufacturers.
THIS IS AN ACTIVE LAWSUIT
Latest NEC Lawsuit Update
As of July 2024, there are 534 active baby formula necrotizing enterocolitis lawsuits pending in Illinois under U.S. District Judge Rebecca R. Pallmeyer. So far, MDL number 3026 has not seen any jury verdicts or global settlements.
Abbott Laboratories is on trial in Missouri, accused of causing NEC in Margo Gill’s premature daughter with Similac and failing to warn about the baby formula’s risks. A St. Louis jury is hearing the case, which is one of hundreds of similar lawsuits.
A new baby formula lawsuit was filed in the NEC MDL by the parents of M.N., a premature infant who tragically passed away after developing NEC. The plaintiffs allege that cow’s milk-based baby formula products from infant formula manufacturers Mead Johnson, including Enfamil Premature Infant Formula and Enfacare, caused the condition.
According to the lawsuit, M.N. was born prematurely on October 3, 2015, at the University of Mississippi Medical Center in Jackson, Mississippi. Shortly after birth, M.N. was fed the defendants’ cow’s milk-based baby formulas. The infant soon developed NEC, a severe intestinal condition that required surgery. Unfortunately, M.N. succumbed to the injuries and passed away on October 29, 2015.
The wrongful death claim asserts that the parents were not informed about the risks associated with feeding premature infants cow’s milk-based baby formula products, including the potential development of NEC and the risk of severe medical complications or death. The plaintiffs argued that if they had known about these risks, they would not have allowed M.N. to be fed the defendants’ baby formula products.
The lawsuit seeks compensation for the financial and economic losses, as well as the emotional harm and distress suffered by the plaintiffs due to their child’s illness and subsequent death.
In a new order issued May 13, 2024, the judge declared that there is no justification for dismissing a plaintiff’s case without prejudice if they are represented by competent counsel in another case. If that counsel becomes unable to represent the plaintiff, substitute counsel can step in, but there will be no grounds to reinstate the previously dismissed case. Henceforth, all such dismissals will be with prejudice, regardless of the request made.
If you have hired more than one lawyer for your NEC lawsuit, it’s important to resolve this issue. Inform all involved lawyers about the situation, decide who you want to represent you, and proceed accordingly. Ensure that you do not dismiss a case filed within the statute of limitations in favor of one where there is uncertainty about meeting the deadline.
Why Are Victims Filing NEC Lawsuits?
Victims are filing NEC lawsuits due to the severe health risks associated with certain baby formulas made from cow’s milk, particularly those baby formula brands like Similac and Enfamil.
Research and cases have shown that cow milk based baby formulas may significantly increase the risk of necrotizing enterocolitis (NEC) in premature infants, a condition that can lead to fatal complications. Plaintiffs argue that the baby formula manufacturers, Mead Johnson and Abbott, failed to provide adequate warnings about this risk, despite evidence suggesting the danger to vulnerable premature infants.
The NEC baby formula lawsuits claim that better warnings could have led parents or caregivers to choose different, safer options for infant nutrition. These legal actions seek compensation for the devastating health outcomes allegedly caused by these formulas.
As the number of affected families grows, these NEC baby formula lawsuits aim to hold formula manufacturers accountable for not sufficiently ensuring the safety of their products for all infants.
Get a Free Legal Case Review Now!Health Implications of NEC
The primary health concern of necrotizing enterocolitis (NEC) in infants is severe intestinal damage, which can be fatal, especially in premature babies. This condition leads to the death of intestinal tissue, and potentially, holes can form in the intestine. Such complications allow bacteria to leak into the abdomen or bloodstream, causing severe infections or sepsis, which can be life-threatening.
Known Injuries and Side Effects
NEC primarily affects the gastrointestinal system of infants, leading to a range of critical symptoms and side effects including:
- Abdominal pain and bloating
- Blood in stools
- Feeding difficulties
- Lethargy
- Instability in body temperature
- Respiratory distress
These symptoms may come on over a few days or appear suddenly in premature babies.
Eligibility for Victims and Legal Procedures
You may be eligible to file an NEC baby formula lawsuit if you are a parent or guardian of an infant who developed necrotizing enterocolitis (NEC) after being fed cow milk-based formulas such as Similac or Enfamil. The lawsuit’s central claim must be that the infant’s NEC condition directly resulted from these formulas, and that the manufacturers failed to provide adequate warnings about such risks.
Am I Eligible to File an NEC Lawsuit?
To determine eligibility for an NEC lawsuit, NEC baby formula lawyers require the confluence of the following three factors:
- Premature baby
- Use of Enfamil or Similac formula
- Diagnosis of necrotizing enterocolitis
Medical documentation of NEC diagnosis, details of formula usage, and the absence of adequate warnings from manufacturers are important in establishing a credible claim.
Key eligibility factors also include the duration and amount of formula intake. Premature babies who were fed these formulas extensively may have a stronger case.
The lack of warnings about the potential risks of NEC at the time of formula use is a crucial aspect, as it forms the basis of claims that manufacturers failed to inform caregivers adequately about the potential health risks of feeding cow milk baby formula to premature babies.
Steps to File an NEC Lawsuit
Filing an NEC baby formula lawsuit, like other personal injury or product liability claims, follows a series of steps to ensure the validity and strength of the claim. The following are the general steps to file an NEC lawsuit:
- Consult with an attorney: It’s crucial to start by consulting with an experienced lawyer who specializes in product liability or personal injury claims. They can provide guidance on the strength of your case, potential compensation, and the litigation process.
- Gather evidence: Collect all pertinent medical records, purchase receipts, and any documentation that shows your child was fed these formulas. This will demonstrate the connection between the formula and your child’s health condition.
- File the complaint: Your attorney will draft a complaint, which is the formal document that starts the lawsuit. It outlines the facts of the case, legal arguments, and the damages being sought.
Potential Compensation and Settlements
A significant verdict was reached in March 2024, when an Illinois jury ordered Mead Johnson to pay $60 million to the mother of a premature infant who died after being fed Enfamil, a cow’s milk-based formula.
This judgment, part of broader litigation concerning NEC risks from such formulas, highlights the potential financial consequences facing manufacturers.
This case’s outcome may influence future settlements and trials in ongoing NEC-related lawsuits, as it represents one of the first substantial awards in this extensive legal battle.
All NEC Lawsuit Updates
July 2024
There are 534 active baby formula necrotizing enterocolitis lawsuits pending in Illinois under U.S. District Judge Rebecca R. Pallmeyer. So far, MDL number 3026 has not seen any jury verdicts or global settlements.
Abbott Laboratories is on trial in Missouri, accused of causing NEC in Margo Gill’s premature daughter with Similac and failing to warn about the baby formula’s risks. A St. Louis jury is hearing the case, which is one of hundreds of similar lawsuits.
In late June, a new baby formula lawsuit was filed in the NEC MDL by the parents of M.N., a premature infant who tragically passed away after developing NEC. The plaintiffs allege that cow’s milk-based products from infant formula manufacturers Mead Johnson, including Enfamil Premature Infant Formula and Enfacare, caused the condition.
According to the lawsuit, M.N. was born prematurely on October 3, 2015, at the University of Mississippi Medical Center in Jackson, Mississippi. Shortly after birth, M.N. was fed the defendants’ cow’s milk-based baby formulas. The infant soon developed NEC, a severe intestinal condition that required surgery. Unfortunately, M.N. succumbed to the injuries and passed away on October 29, 2015.
The wrongful death claim asserts that the parents were not informed about the risks associated with feeding premature infants cow’s milk-based products, including the potential development of NEC and the risk of severe medical complications or death. The plaintiffs argued that if they had known about these risks, they would not have allowed M.N. to be fed the defendants’ baby formula products.
The lawsuit seeks compensation for the financial and economic losses, as well as the emotional harm and distress suffered by the plaintiffs due to their child’s illness and subsequent death.
June 2024
After two consecutive months of above-average new case volume, May saw a slight decline in new baby formula cases added to the NEC infant formula MDL. Over the past 30 days, only 18 new baby formula lawsuits were reported, bringing the total number of pending cases to 514.
May 2024
In April, the NEC preterm infant formula MDL saw the addition of 43 new cases, continuing a trend of elevated new case volume for the second consecutive month. The current count of pending baby formula lawsuits now stands at 496.
To streamline the service of Short Form Complaints (SFC) to counsel of record and ensure more accurate and transparent reporting, the court has enlisted BrownGreer to implement specific protocols outlined in a recent order. BrownGreer, a legal services firm specializing in mass claims administration and settlement administration, will handle the service and maintenance of SFCs for all cases filed in the MDL.
Plaintiffs in mass tort litigation often hire more than one lawyer, leading to duplicate cases filed by different law firms. The MDJ judge has received several motions and stipulations of dismissal due to these duplicate cases. Some requests seek dismissal with prejudice, while others seek dismissal without prejudice.
In a new order issued May 13, 2024, the judge declared that there is no justification for dismissing a plaintiff’s case without prejudice if they are represented by competent counsel in another case. If that counsel becomes unable to represent the plaintiff, substitute counsel can step in, but there will be no grounds to reinstate the previously dismissed case. Henceforth, all such dismissals will be with prejudice, regardless of the request made.
April 2024
Over the past month, the preterm infant formula NEC class action lawsuit MDL saw an additional 50 new cases filed, marking the highest monthly surge since its inception. This brings the total number of active NEC infant formula cases to 453, a significant rise from 342 cases reported at the beginning of the year.
March 2024
A significant court ruling in Illinois awarded $60 million in an NEC baby formula lawsuit against Mead Johnson. This is the first NEC failure-to-warn lawsuit to go to trial. It concluded with a jury verdict significantly higher than the $25 million sought by plaintiffs, marking a crucial precedent in NEC infant formula litigation.
The infant formula NEC lawsuits MDL has grown to 405 cases, with 16 new baby formula cases added in the last month.
January 2024
The NEC baby formula MDL saw an addition of 47 new cases, representing the second largest monthly increase since the beginning of the MDL.The NEC lawsuits continue to grow as more parents join the litigation, with 389 total cases pending.
December 2023
The NEC preterm baby formula MDL saw a significant increase, with 50 new cases added, one of the highest monthly volumes to date. This surge brings the total number of pending baby formula cases to 339. Earlier in the month, on December 1, the count stood at 290 after an addition of 15 new cases.
November 2023
U.S. District Judge Rebecca R. Pallmeyer in Illinois has chosen four pivotal “bellwether” cases for trial, aimed at evaluating potential jury responses to the evidence. Two of these cases involve both Abbott Laboratories and Mead Johnson, while the others individually target each company.
Three cases involve wrongful death claims due to necrotizing enterocolitis linked to the formulas, and one involves an infant who survived necrotizing enterocolitis (NEC) but underwent multiple surgeries.
Trials are expected to begin in 2024. The outcomes could significantly influence future NEC settlement strategies in this class action.
October 2023
A modest increase in baby formula cases occurred with 12 new NEC lawsuits added, reflecting a steady but slower accumulation in the MDL.
Following a recent status conference, the Judge approved a short extension for the plaintiffs to select bellwether cases.
September 2023
In September, 83 new cases were added to the NEC class action MDL. This raises the total to 263 active cases, significantly up from 97 at the beginning of the year.
Meanwhile, there’s been tension between the plaintiff lawyers and defendants regarding the depositions of treating doctors, crucial for the lawsuits. Defendants, Abbott and Mead, are pushing for the first jury trial to be held in federal court.
After disagreements on the timeline, the court required the parties to present a mutually agreed-upon order detailing an updated discovery timetable and setting a further status conference.
August 2023
The NEC infant formula lawsuit class action has doubled since the start of the year, with 14 new baby formula cases added last month, bringing the total to 205. At this rate, the case count could reach approximately 300 by year-end and potentially 400 by the first bellwether trials.
A significant wrongful death lawsuit, Rowe v. Abbott, was directly filed into the NEC class action MDL. This case involves a child born prematurely in 2007 who tragically passed away from NEC after being fed Abbott’s cow milk-based formula in the NICU.
The NEC baby formula lawsuit alleges that Abbott failed to provide adequate warnings and guidelines for the use of their products in preterm infants, specifically concerning the risks of necrotizing enterocolitis (NEC).
July 2023
11 new cases were transferred to the NEC infant formula class action, bringing the total to 191. The year started with only 90 cases, showing substantial growth in the baby formula litigation.
A significant legal discussion emerged around the statute of limitations, particularly in California where a judge allowed amendment and refiling of sixteen dismissed baby formula cases.
June 2023
The NEC infant formula class action MDL now has 180 active plaintiffs, following the addition of 14 new cases over the last month. Since the beginning of the year, a total of 83 new baby formula cases have been transferred to the MDL, indicating a steady increase in the number of families seeking legal recourse.
May 2023
The U.S. Federal Trade Commission (FTC) is investigating potential collusion among baby formula manufacturers, including Abbott Laboratories, concerning state contract bids for the Women, Infants, and Children (WIC) program. This inquiry also involves sales outside the WIC program and occurs amid disputes over the extent of information Abbott must disclose following a formula shortage.
In Illinois, economic loss claims related to allegedly contaminated Similac were dismissed by a federal judge, who ruled that plaintiffs did not prove the purchased formula was tainted. However, personal injury claims regarding infant illnesses were upheld, focusing legal efforts on these more critical allegations.
Nine new NEC-related lawsuits were added to the class action MDL, totaling 166 pending cases. The lawsuits allege necrotizing enterocolitis (NEC) in premature infants due to the formula.
Electronic discovery remains pivotal, with ongoing disputes over the number of the companies’ custodians as witnesses in pretrial discovery. Mead refused a global compromise, causing the plaintiff’s attorneys to file a motion to compel.
April 2023
12 bellwether candidate cases in the NEC formula MDL are now undergoing case-specific fact discovery as part of the pretrial process. This involves depositions of witnesses and doctors, aiming to provide detailed case insights before selecting 4 cases for the upcoming bellwether trials set for next year.
A significant increase in litigation activity was noted with 35 new cases added to the NEC class action MDL, the highest monthly intake since the start of litigation. The total now stands at 157 cases, with expectations for the first bellwether trial to begin next year.
March 2023
17 new cases have been added to the NEC class action MDL, increasing the total to 122 plaintiffs. This marks the second largest monthly addition since the MDL began.
February 2023
The NEC baby formula class action litigation in the Northern District of Illinois includes 97 pending cases, with 5 new cases added over the last month. The number of cases remains consistent as many NEC cases are either staying in state courts or being remanded back, not being included in the MDL.
January 2023
The judge overseeing the NEC infant formula MDL in the Northern District of Illinois plans to schedule a “science day.” These sessions are commonly used in mass tort MDLs to allow both parties to present and clarify the relevant scientific evidence for the court. The exact date for the NEC science day is expected to be determined during the January monthly status conference.
November 2022
8 cases were selected for the bellwether program in the NEC infant formula class action MDL, with defendants set to choose four additional cases by month’s end. The MDL Judge approved a fact sheet form for plaintiffs in these 12 bellwether cases to complete as the initial step in the discovery process, which will be followed by depositions.
The judge remanded 29 cases to state courts in Pennsylvania due to the involvement of local hospitals, reducing the MDL’s case count to 106.
October 2022
Attorneys from both sides in the NEC class action MDL submitted a list of 66 cases to the judge, from which 12 bellwether candidates will be chosen. Each side picked four cases, and four additional cases will be randomly selected by the court. These 12 will undergo pretrial discovery, after which 4 will be selected for the initial trials, with each side choosing 2.
The judge outlined the selection process and schedule for these bellwether trials, with the final selection due by November 23. This structured approach aims to manage the discovery efficiently and prepare for the staggered trial dates, set 12 weeks apart.
September 2022
Attorneys involved in the NEC infant formula class action have focused on a draft plan for selecting the initial NEC baby formula lawsuit for trial.
The plan, under review by the MDL judge, involves selecting a pool of 12 cases, balanced between those involving Abbott and Mead Johnson, either together or individually. These cases will undergo pretrial processes including discovery and depositions. When discovery is completed, each side will select two cases from this group for the first round of four bellwether trials.
August 2022
The NEC baby formula lawsuit MDL continues, with the Plaintiffs’ MDL Leadership Committee nearing completion of negotiations on discovery protocols and other procedures with the defendants. Meanwhile, about a dozen plaintiffs have successfully had their cases remanded to state courts based on a lack of diversity jurisdiction.
July 2022
Over the last month, 9 new NEC lawsuits were transferred into the Preterm Infant Nutrition MDL, bringing the total to 97 cases. Despite this, many NEC attorneys continue to file additional lawsuits in Illinois state courts.
The federal judge overseeing the NEC baby formula class action has moved to allow direct filings against Abbott and Mead Johnson in the MDL, simplifying the process for new plaintiffs. Previously, cases had to be filed in home districts and then transferred. Mead Johnson objected to this order. Abbott did not object.
June 2022
The NEC MDL saw a significant expansion with 37 new plaintiffs added in its first month, effectively doubling its size.
Progress continues in the NEC class action baby formula lawsuit. Judge Rebecca R. Pallmeyer issued an Order finalizing the appointments of the Plaintiffs’ Lead Counsel, Liaison Counsel, and Steering Committee. With 26 additional cases transferred since May 16th, the total has reached 78 pending lawsuits.
May 2022
The NEC class action baby formula lawsuit (MDL No. 3026) in the Northern District of Illinois is formally underway. Judge Rebecca Pallmeyer held her first status conference on May 19. She is expected to announce her selections for the plaintiffs’ Lead Counsel, Liaison Counsel, and leadership committee within the next two weeks.
There are now 3 baby formula class action lawsuits : a federal NEC class action, state-specific NEC lawsuits filed in Illinois, and a Similac recall class action unrelated to NEC. All baby formula lawsuits are progressing concurrently.
April 2022
A new NEC baby formula class action lawsuit has officially been established in federal court. The Judicial Panel on Multidistrict Litigation (JPML) has consolidated all pending NEC infant formula lawsuits into MDL No. 3026, entitled “In re: Abbott Laboratories, et al., Preterm Infant Nutrition Products Liability Litigation.” This MDL has been assigned to Chief Judge Rebecca R. Pallmeyer in the Northern District of Illinois, a venue favored by plaintiffs’ attorneys, despite attempts by defendants Abbott and Mead Johnson to have the MDL located in Connecticut.
The NEC formula litigation is expanding, with over 40 lawsuits filed against Mead and Abbott in federal courts over the past year, including 12 in March alone. Additionally, a smaller, related class action lawsuit is proceeding in Illinois.
March 2022
Discussions are underway about establishing an NEC baby formula class action in federal courts, potentially housed in Connecticut or Illinois. Abbott favors the District of Connecticut, while NEC plaintiffs’ lawyers are electing the Northern District of Illinois, where Abbott has its headquarters.
The Supreme Court of Illinois consolidated about 20 state NEC formula cases into a single class action under Judge Dennis Ruth in Madison County. This is akin to a mini-MDL at the state court level. Abbott is now pushing to transfer these cases to Cook County (Chicago) or Lake County for convenience.
February 2022
NEC baby formula lawyers petitioned the Supreme Court of Illinois to consolidate all state NEC baby formula lawsuits into one action in Madison County. While Abbott and Mead Johnson, both based in the Chicago area, were granted extensions to respond to the consolidation request, their stance on the consolidation remains uncertain.
Increasing legal pressures on baby formula manufacturers were compounded by a petition filed by consumer protection groups to the FDA. This petition demands a ban on certain additives in infant formulas, citing potential health risks.
Amid ongoing NEC baby formula lawsuits, Abbott faced further challenges as the FDA announced a significant Similac recall affecting thousands of children.
December 2021
Abbott faces a new class action over misleading claims about Similac Pro Advance, paralleling ongoing NEC baby formula lawsuits and underscoring questionable marketing practices. The case, emphasizing consumer deception, could support broader NEC litigation claims.
New research from McMaster University highlights increased risks of NEC and developmental delays in premature infants fed cow-milk-based formula, reinforcing concerns over such products.
Illinois state court sees a surge in NEC infant formula lawsuits, leading to a motion for consolidation similar to an MDL due to the local presence of Abbott Laboratories and Mead Johnson. The motion aims to streamline 33 cases, potentially setting the stage for a national MDL and future comprehensive settlements.
FAQs NEC
What is necrotizing enterocolitis (NEC)?
Necrotizing enterocolitis (NEC) is a serious gastrointestinal problem that primarily affects premature infants. It is a disease of the intestinal tract in which the tissue lining the intestine becomes inflamed, dies, and can slough off.
Is it safe to use formulas associated with NEC risks?
Given the lawsuits and ongoing research linking certain formulas to NEC, it is advisable for parents to consult with healthcare providers about safer feeding options for at-risk preterm infants.
How long does it take to resolve an NEC lawsuit?
The timeline for an NEC lawsuit resolution can vary significantly. Factors include the complexity of the case, the number of parties involved, and ongoing litigation dynamics. Some cases may settle quickly, while others, particularly those part of multidistrict litigation, may take years.
How can I find an attorney for an NEC lawsuit?
With our assistance, locating a lawyer skilled in handling an NEC baby formula lawsuit is easy. Simply complete our contact form, and upon submission, a member of our team will promptly contact you to link you with a seasoned attorney tailored to your case. Reach out to us today without delay to secure the expert legal support and lawyer you need.
File a NEC Lawsuit
You may be entitled to compensation if you’ve experienced health problems from using a specific baby formula. Reach out to see if you qualify.
On This Page
Case Status: Ongoing (Mead Johnson to pay $60 Million in Illinois Verdict)
Defendants: Abbott Laboratories, Mead Johnson
Injuries: necrotizing enterocolitis (NEC) complications and death
